Breakthrough Discovery: INSPIRIS RESILIA Aortic Valve Approved by the FDA
By Adam Pick on August 9, 2018 - This blog is sponsored by Edwards Lifesciences.
In case you missed it… The INSPIRIS RESILIA heart valve is now available in the United States. This is an important development for patients who require a more resilient tissue valve for the replacement of their native or prosthetic aortic valve.^
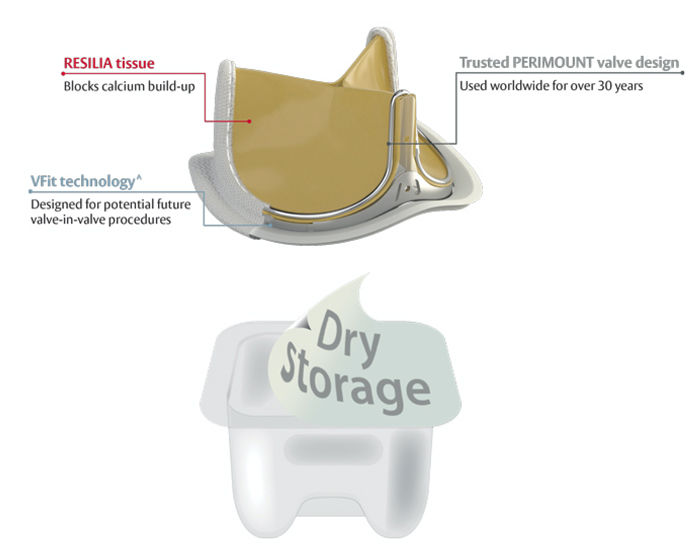
This revolutionary aortic valve (pictured below) is the first in a new class of so-called resilient heart valves introduced by Edwards Lifesciences. It has a new tissue that is better at blocking calcium and a first of its kind expandable valve frame designed for potential future valve-in-valve procedures.*^ And it is built on the proven Carpentier-Edwards PERIMOUNT valve design – which has helped over one million patients worldwide.
To educate our community about this new valve – which took 12 years to develop – I interviewed Dr. Lars Svensson, Chairman of the Heart & Vascular Institute at the Cleveland Clinic, and a world-renowned heart valve specialist. Dr. Svensson was Co-Principal Investigator for the COMMENCE trial that evaluated this new RESILIA tissue technology.
So you know, Dr. Svensson has successfully treated many patients in our community including Connie Taylor, Katherine Wardle and Daniel Conrad.

Why is the FDA approval of the INSPIRIS valve so important for the advancement of valve therapy?
“There are three aspects that are important,” replied Dr. Svensson. “First, the valve has new anti-calcification technology which reduces calcification.* Second, the valve is stored ‘dry’ unlike other valves. Third, the valve is designed to expand during potential future valve-in-valve procedure with a transcatheter aortic valve (TAVR) insertion.”
Let’s examine each of these important points:
1. Blocks Calcium Build-up. The INSPIRIS valve uses the RESILIA tissue, a new type of bovine (cow) tissue with special technology that blocks calcium build-up on the valve.
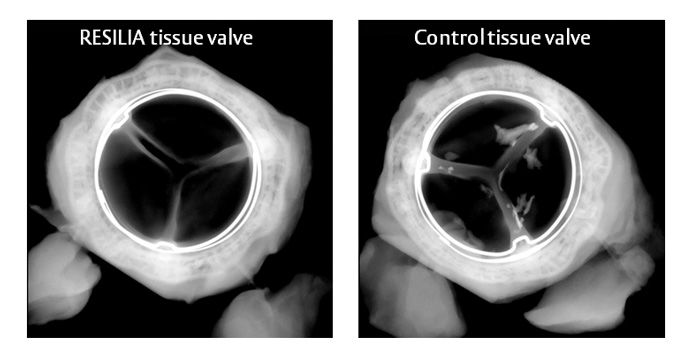
Reducing calcium build-up is important because calcium build-up is the main cause of tissue valve failure. The new RESILIA tissue has demonstrated significantly less calcium build-up in animal studies.*
Here are some radiographic examples of explanted valves from the animal study which showed less calcium build-up.(1)
2. Dry Storage. If you didn’t know… Most tissue valves are stored in aldehyde liquid which attracts calcium. Thanks to the unique RESILIA tissue, the INSPIRIS valve can be stored dry thereby preventing further exposure to the aldehydes.
3. VFit Technology.^ With the approval of TAVR for valve-in-valve procedures in recent years, I was excited to learn that the INSPIRIS valve comes with VFit technology that provides the first-of-its-kind expandable frame specifically designed for potential future valve-in-valve procedures.
What is the number one patient benefit of the INSPIRIS valve?
Dr. Svensson shared, “The number one benefit of the INSPIRIS RESILIA valve is less calcium build-up.* Patients with biscuspid aortic valves, active lifestyles, and those who do not want mechanical valves could benefit from this new valve.”
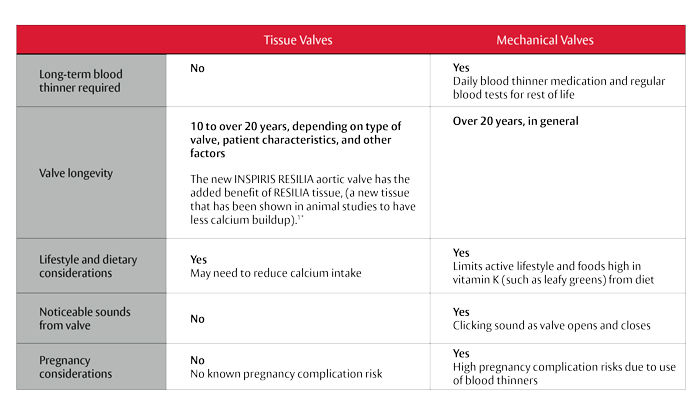
Tissue vs Mechanical Valve Considerations
A key selection criteria for patients going through the valve selection process is durability. The more durable a valve is, the less chance a re-operation will be required.
Mechanical valves are designed with durable materials that may last longer. However, patients with mechanical valves require a lifelong treatment of anticoagulants (blood thinners) to avoid clots on the valve.
Tissue valves, on the other hand, do not require the use of blood thinners and typically last between 10 to 20 years depending on the type of valve, patient characteristics and other factors. That said, the INSPIRIS valve with the more resilient tissue and expandable valve frame may be an attractive benefit for patients who do not want to be on blood thinners for the rest of their life.*
Here are some additional considerations when comparing tissue and mechanical valves:
How are patients with the INSPIRIS valve doing?
“Almost 700 patients were enrolled in the FDA trial, known as the COMMENCE aortic trial, which supported the approval of the INSPIRIS valve,” Dr. Svensson responded. “There have been no structural failures of the study valve during the trial out to 3 years which we reported at the recent American Association for Thoracic Surgery Conference, and hence we did not have any re-operations due to structural valve failures.” This is obviously an encouraging result for a breakthrough technology.
A new resilient valve for patients!
This is a sensational development for patients with aortic valve disease. A new resilient tissue valve that reduces calcium build-up and is specifically designed for potential future valve-in-valve procedures is extremely promising.
Many thanks to Dr. Lars Svensson for sharing his clinical experience and research specific to the INSPIRIS valve with our community.
In addition, I would like to congratulate Edwards Lifesciences for the FDA approval of the INSPIRIS valve. We are optimistic that it will help many patients in our community!
Keep on tickin!
Adam
* RESILIA tissue tested against commercially-available bovine pericardial tissue from Edwards in a juvenile sheep model.1 RESILIA tissue has not been studied for long-term results in patients.
^ VFit technology has not been observed in clinical studies to establish safety and effectiveness for use in valve-in-valve procedures. VFit technology is available on sizes 19-25 mm.
Reference:
1. Flameng et al. A randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J Thorac Cardiovasc Surg. 2015;149:340–5.
Important Risk Information: INSPIRIS RESILIA Aortic Valve
Indications: For use in replacement of native or prosthetic aortic heart valves. Contraindications: There are no known contraindications with the use of the INSPIRIS RESILIA aortic valve. Complications and Side Effects: Thromboembolism, valve thrombosis , hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, any of which could lead to reoperation, explantation, permanent disability, and death. Warnings: DO NOT ADJUST THE VALVE DIAMETER BY EXPANDING THE BAND PRIOR TO/OR DURING IMPLANTATION OF THE SURGICAL VALVE. The expandable band is not designed to allow for compression or expansion during implantation of the surgical valve. This will cause damage to the valve and may result in aortic incompetence. DO NOT PERFORM STAND-ALONE BALLOON AORTIC VALVULOPLASTY PROCEDURES ON THIS VALVE FOR THE SIZES 19 – 25 mm as this may expand the valve causing aortic incompetence, coronary embolism or annular rupture. Valve-in-Valve procedures in an INSPIRIS valve should be performed according to the combinations in the SAPIEN XT IFU. Other combinations have not been evaluated and may result in the embolization of transcatheter devices anchored within or result in annular rupture.
CAUTION: Federal (United States) law restricts these devices to sale by or on the order of a physician. See instructions for use for full prescribing information, including indications, contraindications, warnings, precautions and adverse events.
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, INSPIRIS, INSPIRIS RESILIA, PERIMOUNT, RESILIA, SAPIEN, and SAPIEN XT are trademarks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.
© 2018 Edwards Lifesciences Corporation. All rights reserved. PP-US-3046 v1.0

|
Tutabella says on August 9th, 2018 at 3:12 pm |
|
Thats amazing. I should have another 5 yrs left on this tissue valve. Hopefully these will be widely available when I need it. |
 |
|
Adam says on August 9th, 2018 at 3:58 pm |
|
I agree Tutabella. The new bovine tissue that reduces calcium. The dry storage. The VFit technology. It’s all quite amazing. Fyi, Dan Vechiola is getting his aortic valve replaced using the INSPIRIS valve at Northwestern Medicine in Chicago. You can learn more about it from Dan at https://www.heart-valve-surgery.com/journals/user/danvechiola. Here’s a picture he took of the valve during his consult with Dr. McCarthy. Pretty neat, right? https://uploads.disquscdn.com/images/89c40dacb89f7e42cf4a452a4490ab9e2c2273159f90eb1bf0c76dff3f0592a2.jpg |
 |
|
Margreth says on August 10th, 2018 at 5:41 pm |
|
Hi Adam, first of all, many thanks for your great newsletter. Thanks to you, I now feel much less frightened to having my Aortic Valve replaced. And this INSPIRIS RESILIA heart valve sounds interesting and promising. Do you know, if any cardiologist in the Los Angeles area is familiar with it ? I understand, Cedar Sinai is one of the better hospitals in the country for aortic valve replacement. |
 |
|
Adam says on August 13th, 2018 at 10:18 am |
|
Hi Margreth! To learn which doctors specialize in the INSPIRIS RESILIA valve, you may want to contact Edwards Lifesciences, the company that makes that valve. Here’s a link to help you do that – https://www.edwards.com/aboutus/ContactUs. Quick question… Have you thought about getting a second opinion about your valve? That might help you get comfortable regarding the management of your valve disease. Thoughts? Adam |
 |
|
Margreth says on August 17th, 2018 at 3:23 pm |
|
Thank you, Adam. You are right, I should get a second opinion and will look for a good doctor to do that. |
 |
|
indoamericanhelth says on August 21st, 2018 at 7:04 am |
|
important and useful story thanks for sharing information http://www.indoamericanhealth.com |
 |
|
shirlag1 says on August 30th, 2018 at 2:05 pm |
|
Hi Adam, I contacted Edwards Lifesciences to learn which doctors specialize in the Inspiris Resilia valve but I didn’t get anywhere with that. Do you have any other suggestions concerning this? Maybe I just need more specifics in how to contact and speak with Edwards Lifesciences. I wrote to them and also called them. Someone called me back but her advice was to talk to my cardiologist about this. I knew right away that he probably wouldn’t know since he’s not a valve specialist cardiologist so I asked what to do if that were the case. She did say to call her back if I didn’t find the information I was seeking. It was really frustrating. Shouldn’t a company know where they are distributing their own product? I know they must. I hope I can find out a list of surgeons in my area and outside my area soon. |
 |
|
Adam says on August 31st, 2018 at 7:43 am |
|
Hi there, Sorry to hear about the frustration you experienced. When you called Edwards, did you try calling 800-4-A-HEART? Or, did you fill out a form on their website? Or, both? |
 |










