Breaking News: New Medtronic Evolut Low-Risk TAVR Clinical Trial Results
Written By: Allison DeMajistre, BSN, RN, CCRN
Medical Expert: Michael Reardon, MD, Professor of Cardiovascular Surgery at Houston Methodist
Reviewed By: Adam Pick, Patient Advocate, Author & Website Founder
Page Last Updated: May 30, 2025
Once again, we’re hearing more promising news about low risk transcatheter aortic valve replacement (TAVR) clinical trial.
Medtronic, a healthcare technology company and global leader in heart valve manufacturing, recently announced its 4-year outcomes from the Medtronic Evolut Low Risk TAVR Clinical Trial, and the research continues to yield encouraging results.
So you know, the U.S. Food and Drug Administration (FDA) first approved TAVR procedures in 2011 for patients at extreme risk who are not candidates for surgical aortic valve replacement (SAVR) with severe aortic stenosis. In 2016, intermediate surgical risk patients were approved for the TAVR procedure. The Medtronic Evolut Low Risk TAVR Clinical Trial, which evaluates TAVR against SAVR for low surgical risk patients, helped extend the FDA’s TAVR approval for low-risk patients in 2019. However, even with the FDA approval, Medtronic continues to follow the patients involved in the study and report the statistical results each year.
For this reason, I was very excited to be able to connect with Dr. Michael Reardon, Professor of Cardiovascular Surgery at Houston Methodist in Houston, Texas, and Co-Principal Investigator of the Medtronic Evolut Low Risk TAVR Clinical Trial while at the Transcatheter Cardiovascular Therapeutics (TCT) Conference in San Francisco, California. Dr. Reardon was at TCT specifically to share the late-breaking results of the Medtronic Evolut Low Risk TAVR Clinical Trial, and he was kind enough to share the details with us.
Key Learnings About The 4-Year Results From The Evolut Low Risk Clinical Trial
- Interest in TAVR remains very high for aortic stenosis patients. “Everybody is interested in TAVR,” Dr. Reardon said. “The reason people are so interested in TAVR is because it behaves differently than surgery. As a heart surgeon, I can tell you we do great surgery. We get good results, but TAVR is far less invasive,” he said. TAVR risks may include, but are not limited to, death, stroke, damage to the arteries, bleeding and need for permanent pacemaker. For some patients, the Medtronic TAVR procedure risks may outweigh the benefits. Please talk to your doctor to decide whether this therapy is right for you.
- Dr. Reardon told us that his average patient goes home the day after a TAVR procedure, and within one week, that average patient returns to entirely normal activity. The bigger question is, how will TAVR patients do over time? “That’s the data we’re looking for in this trial to help educate our patients and physicians in making this difficult lifetime management decision.”

- The latest study results evaluated several key indicators for TAVR within low risk populations. For those unfamiliar with the clinical trial, Dr. Reardon told us exactly what they were studying. “We took people we thought were at low risk and randomly assigned them to either having TAVR with the Medtronic valve or surgery where the surgeon got to pick the valve,” he said. The patients are followed year after year, and they watch for several different things, including:
- Clinical outcomes
- How well the valve is working
- Failing valves
- Long-term clinical outcomes, unexpected complications
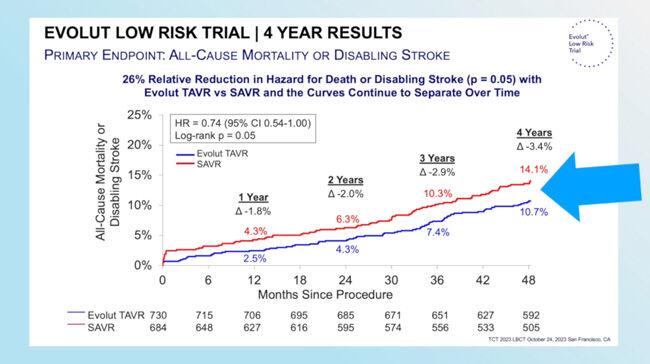
- “The information we have so far for the Medtronic TAVR valve is that it not only maintains the early advantage, but its advantage widens over time,” Dr. Reardon said.
- This advantage can be seen in what Dr. Reardon shared about the definitive “Top Findings” from this research. The Medtronic TAVR had significantly better hemodynamics (blood flow) after one-year compared to surgery and has remained that way through four years. Additionally, Dr. Reardon said they’ve shown good outcomes, with the all-cause mortality and disabling stroke rates between TAVR and SAVR widening yearly for the first four years.1
- The Medtronic Evolut Low Risk TAVR Clinical Trial is following patients for ten years; typically, these studies are reported at one, two, five, and ten years. However, Dr. Reardon told us they’ve been reporting results yearly and plan to continue reporting each year through the end of the trial. “This is important information, not just for patients, but for the physicians who care for them and help make lifetime decisions in treating their aortic stenosis.”
- The statements and information presented herein may be the opinion of the individual physician featured and is not intended to constitute medical advice.
Thank you, Dr. Reardon, Houston Methodist, and Medtronic!
On behalf of our patient community, thank you to Dr. Michael Reardon for sharing this incredible news from the Medtronic Evolut Low Risk TAVR Clinical Trial. We would also like to thank Medtronic for its ongoing research into the revolutionary technology and life-changing devices that continue to transform the lives of heart valve patients worldwide!
Related Links:
- See the Medtronic TAVR Procedure Educational Microsite
- Research 50 Leading Heart Teams the Specialize in Medtronic TAVR
- Free eBook: What 7 Facts Should You Know About TAVR?
Keep on tickin!
Adam
P.S. For the deaf and hard-of-hearing members of our patient community, I have provided a written transcript of this interview below.
References:
1. Reardon M, et al. Transcatheter Versus Surgical Aortic Valve Replacement in Aortic Stenosis Patients at Low Surgical Risk: 4-Year Outcomes from the Evolut Low Risk Trial. Presented at TCT; October 2023.
Video Transcript:
Adam Pick: Hi, everybody. It’s Adam with heartvalvesurgery.com and we are at the Transcatheter Cardiovascular Therapeutics Conference in San Francisco, California. I am thrilled to be joined by Dr. Michael Reardon who is a professor of cardiovascular surgery at Houston Methodist in Houston, Texas. Dr. Reardon is also the national surgical director of the Evolut Low Risk TAVR Clinical Trial. Dr. Reardon, great to see you again.
Dr. Reardon: Adam, thanks for having me on.
Adam Pick: Yeah, so we’re here at TCT learning lots of new things about valvular disease and treatment. I’m curious to know what brings you to TCT today.
Dr. Reardon: Well, specifically, I came here to talk about the late break in clinical trial for the results of the low-risk trial. Everybody is interested in TAVR. The reason they’re interested in TAVR is it behaves differently than surgery. As a heart surgeon, I can tell you we do great surgery. We get good results, but TAVR is far less invasive. My average patient goes home the next day. My average patient at one week is back to completely normal activity. The real question is, will this early advantage hold up over time? That’s the data we’re looking for in this trial to help educate our patients and physicians in making this difficult lifetime management decision.
Adam Pick: Maybe could you share for the patients who may not be familiar with clinical trials, first, what did you study?
Dr. Reardon: What we did is we took people that we thought were at low risk and we randomly assigned them to either having TAVR the Evolut valve or surgery, the surgeon got to pick the valve. Then we follow them year by year by year. We watch for a lot of different things. We watch for clinical outcome. We measure how well the valve is working. We look to see if any valves are failing. We look to see if there’s any long-term unexpected complications that come up. Again, the information we have so far for this valve is it not only maintains this early advantage, but this advantage widens overtime.
Adam Pick: Yeah, so let’s talk about that, the findings. For the patients out there, what would you say are the definitive Top 3 findings of this study?
Dr. Reardon: I think the definitive Top 3 things we found out about this valve is that when we look at structural valve deterioration, the valves starting to fail, it occurs more often at a moderate or severe level in surgery than it did in TAVR with the Evolut valve. We also showed if that happened, whether you had TAVR or surgery, it doubled your risk of dying and doubled your hospitalization. We also showed that the hemodynamics, which is the forward flow of how well the valve is working or how close it is to a normal valve, is superior to surgery. Then finally, we showed that these really good performance metrics have now translated to really good outcome metrics. All-cause mortality and disabling stroke is widened every year for the first four years. Then the curve, looking at it, it looks like it’s going to continue to widen.
Adam Pick: First of all, fantastic results and findings. This doesn’t stop today. It continues on. Is that correct?
Dr. Reardon: That’s correct. The study will be a ten-year study. Typically, studies are reported one, two, five, and ten years. We think in this low-risk population, patients and doctors deserve to know more often. We so far are reporting our results every year and we plan to report our results every year through the lifetime of the trial. This is important information, not just for patients, but for the physicians who care for them helping them make a lifetime decision in treating their aortic stenosis.
Adam Pick: Dr. Reardon, on behalf of patients at heartvalvesurgery.com, patients all over the world, I cannot thank you enough for all the great work that you’re doing there at Houston Methodist in Houston, Texas and on the Evolut Low Risk TAVR Clinical Trial. Thanks so much.
Dr. Reardon: Adam, thank you for bringing this information to our patients. It’s something that I think is helpful to them in making their decision.





