Take Control of Aortic Stenosis Without Open Heart Surgery
Did you know more than 600,000 symptomatic severe aortic stenosis patients have received Medtronic TAVR, a minimally-invasive treatment that restores normal life without surgery?1
Learn More Find DoctorsTake Control of Aortic Stenosis
Without Open Heart Surgery
Did you know more than 600,000 symptomatic severe aortic stenosis patients have received Medtronic TAVR, a minimally-invasive treatment that restores normal life without surgery?1
Learn More Find DoctorsThe educational information provided on this page is sponsored by Medtronic. Important safety information.
“Aortic stenosis is a deadly disease that is under-treated.
TAVR has been a game changer for my patients.”
-- Dr. Ashish Aneja, Associate Director, Noninvasive Cardiovascular Imaging
The MetroHealth System, Cleveland, Ohio
-- Dr. Ashish Aneja, Associate Director, Noninvasive Cardiovascular Imaging
The MetroHealth System, Cleveland, Ohio
Patients diagnosed with aortic stenosis need to understand the symptoms, the mortality risks and their treatment options. In this video, Dr. Ashish Aneja shares important research about aortic stenosis along with a meaningful discussion about treatment options including minimally-invasive therapies like transcatheter aortic valve replacement (TAVR).
Over 600,000 Medtronic TAVRs Have Helped
Symptomatic Severe Aortic Stenosis Patients1
Backed by proven performance and continually advancing technoloy, the Medtronic TAVR procedure has treated over 600,000 patients worldwide since 2014.1
“New clinical trial research shows that the
minimally-invasive Medtronic TAVR outperforms
surgery with sustained valve performance.”2
-- Dr. Michael Reardon, Chairman of Cardiovascular Research,
Professor of Cardiovascular Surgery at Methodist Health, Houston, Texas

“If I didn’t have the Medtronic TAVR procedure, I don’t think I would be playing golf and gardening. TAVR gave me a new lease on life.”
-- Gary, 69, Aortic Stenosis Patient

“If I didn’t have the Medtronic TAVR procedure, I don’t think I would be playing golf and gardening. TAVR gave me a new lease on life.”
-- Gary, 69, Aortic Stenosis Patient
Which Patients Qualify? How Does TAVR Work?
What Do Patients Experience During TAVR?
In this video, Dr. Michael Deeb, Inaugural Herbert Sloan Professor of Cardiac Surgery at University of Michigan Health, explains how TAVR works, which patients qualify for TAVR, what patients experience during TAVR, and the durability of this minimally-invasive procedure.
Find “Heart Teams” That Specialize In TAVR
A “Heart Team” is a specialized care team that includes cardiologists, cardiac surgeons, imaging specialists, anesthesiologists, valve program coordinators, and advanced practice providers that specialize in aortic stenosis treatment options including TAVR. Please find below fifty Heart Teams across the United States.
TAVR eBook To Help You Learn More
To learn more about the Medtronic TAVR procedure, click here to download our new TAVR eBook, “Medtronic TAVR Procedure: What 7 Facts Should You Know?” You can share this eBook with your family and friends.

Questions & Answers About Medtronic TAVR

Question 1: Is the Medtronic TAVR approved by the FDA?
Answer: The U.S. Food and Drug Administration (FDA) has approved the Medtronic TAVR procedure as a safe-and-effective therapy for the treatment of symptomatic severe aortic stenosis in patients who are at high-risk, intermediate-risk and low-risk for death or major complications associated with open-heart surgery to replace damaged heart valves.
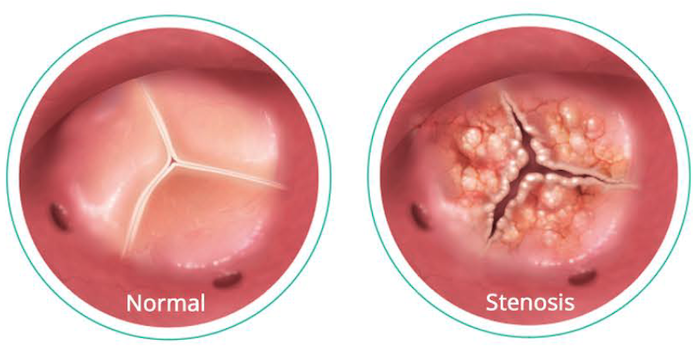


Question 2: Is the Medtronic TAVR available to all patients with aortic valve disease?
Answer: The Medtronic TAVR procedure is currently approved for (i) patients with heart disease due to symptomatic severe native aortic stenosis and (ii) patients with a failing surgical aortic valve who are at high risk or extreme risk for complications during surgery. Talk to your doctor to see if Medtronic TAVR is right for you. Contact a TAVR Heart Team.

Question 3: How long are patients in the hospital after a TAVR procedure?
Answer: After a TAVR procedure, most patients spend a few hours in the intensive care unit (ICU) before transferring to a patient room. Typically, patients begin walking the same day as their Medtronic TAVR procedure. Most patients who had the Medtronic TAVR procedure go home the next day and get back to their everyday activities faster than open heart surgery.3

Question 4: Is a Medtronic TAVR durable compared to surgical heart valves?
Answer: According to a clinical study, the Medtronic TAVR is the only TAVR with proven durability of less structural valve deterioration versus surgical valves at 10 years.4
The Medtronic TAVR had superior hemodynamics (blood flow) at one year compared to surgery and remained excellent through four years in a clinical study. In that same study, the Medtronic TAVR also showed improved outcome metrics including all-cause mortality and disabling stroke versus surgery.5

Question 5: How do patients feel after a TAVR procedure?
Answer: Most patients start feeling better right away. This is because your heart valve is now working properly. Some patients may take longer to feel better.
After receiving a Medtronic TAVR, patients have reported benefits like:
- Having more energy
- Breathing normally
- Experiencing less pain
- Experiencing fewer symptoms
- Feeling less anxious
After the procedure, most patients can take care of themselves better and go back to everyday activities.
Most medical procedures have risks. The most serious risks of the Medtronic TAVR procedure are:
- Death
- Stroke
- Serious damage to the arteries
- Serious bleeding (a bleeding event that requires a blood transfusion)
- Need for permanent pacemaker
The chance of an adverse event from the TAVR procedure depends on many factors including your underlying medical conditions.
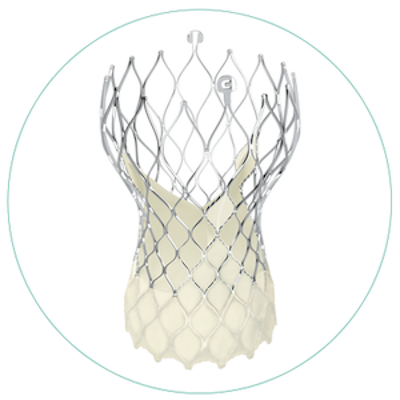
Question 6: What makes the design of a Medtronic TAVR unique?
Answer: The self-expanding Medtronic TAVR provides patients excellent hemodynamics (blood flow) across their aortic valve, which can help keep patients alive and out of the hospital.6
The super annular metal frame is a blend of nickel and titanium (called nitinol). This material allows the frame to shape itself to the patient’s anatomy. The tissue leaflets are positioned super annular (above the patient's diseased valve) and are made from pig heart tissue.
Question 7: Is stroke a risk associated with TAVR?
Answer: Stroke is a risk associated with TAVR and open heart surgery. Initially, there was a higher stroke rate for TAVR compared to open heart surgery but that is no longer accurate. The current stroke rate for TAVR is between 2% to 3%.7
Talk to your physician about the benefits, risks and adverse events associated with TAVR. To find a medical team that specializes in TAVR, click here.
Risk Statements
- TAVR risks may include, but are not limited to, death, stroke, damage to the arteries, bleeding and need for permanent pacemaker.
- For some patients, the Medtronic TAVR procedure risks may outweigh the benefits. Please talk to your doctor to decide whether this therapy is right for you.
- The statements and information presented herein may be the opinion of the individual physician featured and is not intended to constitute medical advice.
Additional Questions?
HeartValveSurgery.com is a patient advocacy group that is not trained or capable of providing medical advice.
HeartValveSurgery.com encourages you to discuss your questions with your medical team. If you are not getting information that you are comfortable with then you may want to get a second opinion to validate and support the next steps in your treatment process.
Additional Questions? HeartValveSurgery.com is a patient advocacy group that is not trained or capable of providing medical advice. HeartValveSurgery.com encourages you to discuss your questions with your medical team. If you are not getting information that you are comfortable with then you may want to get a second opinion to validate and support the next steps in your treatment process.
References
- Medtronic data on file.
- Reardon M, et al. Transcatheter Versus Surgical Aortic Valve Replacement in Aortic Stenosis Patients at Low Surgical Risk: 4-Year Outcomes from the Evolut Low Risk Trial. Presented at TCT; October 2023.
- Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. New England Journal of Medicine, 2019.
- Thyregod HGH, Jorgensen TH, Ihlemann N, et al. Transcatheter or surgical aortic valve implantation: 10-year outcomes of the NOTION trial. Eur Heart J. Feb 7 2024.
- Forrest JK, Deeb GM, Yakubov SJ, et al. Evolut Low Risk Trial Investigators. 4-Year Outcomes of Patients With Aortic Stenosis in the Evolut Low Risk Trial. J Am Coll Cardiol. 2023 Nov 28;82(22):2163-2165.
- Yakubov S, et al. Five-Year Incidence of Bioprosthetic Valve Dysfunction in Patients Randomized to Surgery or TAVR: Insights From the CoreValve US Pivotal and SURTAVI Trials. Presented at CRT; February 2023.
- Carroll JD, Mack MJ, Vemulapalli S, et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. Journal of the American College of Cardiology. 2020/11/24/ 2020;76(21): 2492-2516. Based on 30-day stroke rates from Transcatheter Valve Therapy Registry.
Medtronic CardioVascular Technical Support - Toll-free: (877) 526-7890 or Email: rs.structuralheart@medtronic.com
