Clinical Trial Update: SUMMIT Now Enrolling Patients to Evaluate Transcatheter Mitral Valve Replacement (TMVR)
Written By: Adam Pick, Patient Advocate, Author & Website Founder
Medical Experts: Gorav Ailawadi, MD, Chief of Cardiac Surgery at Michigan Medicine, and Jason Rogers, MD, Professor of Cardiovascular Medicine at UC Davis Health
Page last updated: November 29, 2022; This post is sponsored by Abbott.
While transcatheter aortic valve replacement (TAVR) has become ubiquitous in valvular therapy during the past 10 years, we seldom hear about transcatheter mitral valve replacement (TMVR).
One reason is that TMVR has yet to receive a Food & Drug Administration (FDA) approval in the United States. While there has been much interest and investment for the development of TMVR, we have yet to witness a successful device system that has yielded an FDA approval.
This may be about to change as the SUMMIT Clinical Trial is now enrolling patients to evaluate the safety and the effectiveness of a new TMVR device.
What Is The SUMMIT Clinical Trial?
The primary objective of the SUMMIT Clinical Trial is to study the Tendyne™ TMVR device for patients who have been diagnosed with mitral regurgitation or have severe mitral annular calcification, who are not good candidates for mitral valve surgery (comorbidities, frailty, etc). The Tendyne system replaces the patient’s defective valve with a new bioprosthetic valve.
The Tendyne™ procedure does not require an incision to open the patient’s sternum. In addition, patients are not required to go on the heart-lung machine during a Tendyne™ procedure. Abbott is the manufacturer of the Tendyne™ device and the sponsor of the SUMMIT Clinical Trial.
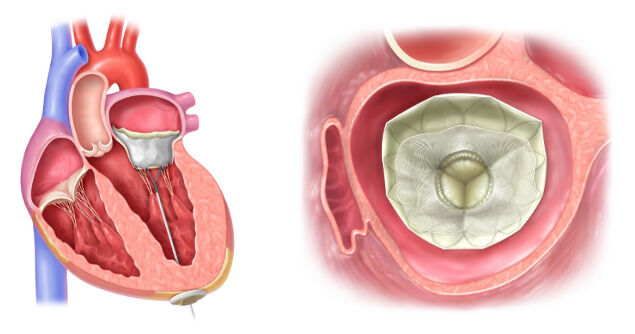 The Tendyne™ Device Implanted in the Mitral Valve
The Tendyne™ Device Implanted in the Mitral Valve
Why Is The SUMMIT Clinical Trial So Important?
The SUMMIT Clinical Trial seeks to evaluate the Tendyne™ TMVR System in treating patients who are not appropriate for surgery and have limited treatment options.
 Dr. Gorav Ailawadi
Dr. Gorav Ailawadi
According to Dr. Gorav Ailawadi, the national co-principal investigator of the SUMMIT Clinical Trial and chief of cardiac surgery at Michigan Medicine, “There is no question that the Tendyne™ system could be a ‘Game Changer’ for mitral valve patients who have limited alternatives. If we can eliminate mitral regurgitation in patients who are at high surgical risk, without stopping the heart, the Tendyne™ device could be transformative.”
This is important research since approximately 70% of people diagnosed with mitral regurgitation are not treated with mitral valve therapies today – yet, are in need of treatment and symptom relief. This trial has set a high bar for the Tendyne™ system. The trial will randomize patients between Tendyne and the transcatheter mitral repair system (MitraClip™).
What Impact Might The SUMMIT Clinical Trial Have On The Future Of Mitral Valve Therapy?
The SUMMIT Clinical Trial could be critically important for future therapies related to mitral regurgitation as patients want safe, less-invasive treatments, shorter hospital stays, and faster recoveries.

Dr. Jason Rogers
According to Dr. Jason Rogers, the national co-principal investigator of the SUMMIT Clinical Trial and professor of cardiovascular medicine at UC Davis Health, “If successful, the SUMMIT Clinical Trial could generate evidence that would open the doors for a new therapy that is not currently available to mitral valve patients.”
Who Can Enroll In The SUMMIT Clinical Trial?
The SUMMIT Clinical Trial will enroll approximately 800 patients at up to 80 sites in the United States, Canada and Europe to evaluate the Tendyne™ device in patients who may not be appropriate for mitral valve surgery. The trial is now enrolling patients.
Click here to learn more about the SUMMIT Clinical Trial.
Keep on tickin!
Adam






